A cooling curve is a line graph that represents the change of phase of matter typically from a gas to a solid or a liquid to a solid the independent variable x axis is time and the dependent variable y axis is temperature.
Independent variable of heating and cooling curve of water.
Heat up the water.
Different substances have different melting points and boiling points but the shapes of their heating curves are very similar.
The initial point of the graph is the starting temperature of the matter here noted as the pouring temperature.
As heat is steadily added to the ice block the water molecules will begin to vibrate faster and faster as they absorb kinetic energy.
I predict that as the time increases the temperature of the water will increase due to the heat expelled from the bunsen burner or other heat source and for cooling i would put.
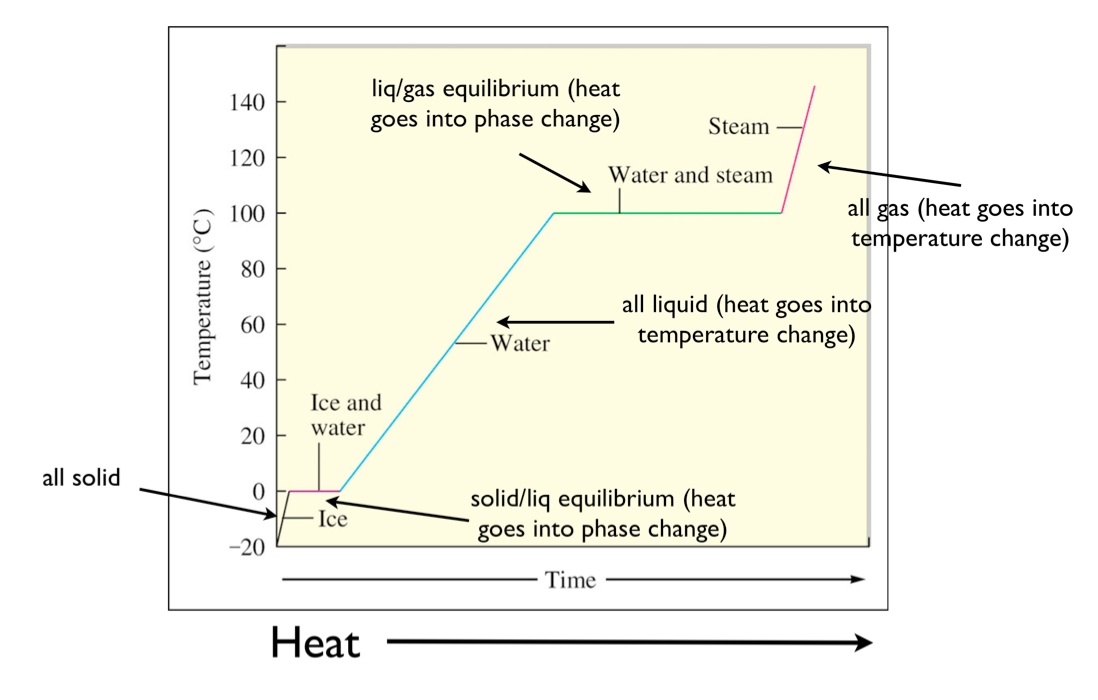
Looking from left to right on the graph there are five distinct parts to the heating curve.
We have been given a physics assignment about the heating and cooling curves of water.
We had to heat ice in a hot water bath over a bunsen burner and record the time every 30 seconds until it boiled and then in a freezer the same way it was just a hypothetical experiment.
Ensure the thermometer is about 2cm above the bottom of the beaker.
Fill an empty beaker with exactly 150ml of water check side scale of beaker set up apparatus as shown above.
Volume is the independent variable.
5 0 minutes 600 seconds temperature of the room.
The dta curve of quartz therefore shows a small sharp endothermic peak that usually occurs close to 573 c on the heating curve because of the reaction α quartz β quartz because of the reversibility of this reaction a comparable exothermic peak will be recorded on the cooling curve if this is determined see curves 1 and 2 fig.
For example this is the heating curve for iron a metal that melts at 1538 c and boils at 2861 c.
Light the bunsen burner and put on a blue flame.
If you are using a bunsen burner for the heating curve i would put something like.
The diagram shows a cooling curve for salol.
For water this temperature is 100 c because the boiling point for water is 100 c.
Notice that the temperature stays the same during the state change freezing and this is the melting point or freezing point of the salol.
Time interval of cooling.
Solid ice is heated and the temperature increases until the normal freezing melting point of zero degrees celsius is reached.
Analysis of a heating curve.
Independent dependent and controlled variables.
Below is an example of a cooling curve used in castings.
Heating curve of water the phase transitions of water.